The global electric vehicle industry keeps its eyes open for any new research in lithium batteries. These research advancements will further enhance consumers’ faith in electric vehicles if implemented at the manufacturing level. One such research development has recently come up from the University of California San Diego (UCSD) that caught the eyeballs of the industry.
The researchers at the UCSD have developed lithium batteries with enhanced performance in extreme cold and hot temperatures. These batteries address two crucial aspects, i.e. high energy density and an all-climate operation simultaneously. Also, they have the potential to allow electric vehicles to cover more distance on a single charge in cold climates and reduce the need for a cooling mechanism to prevent them from overheating in high temperatures.
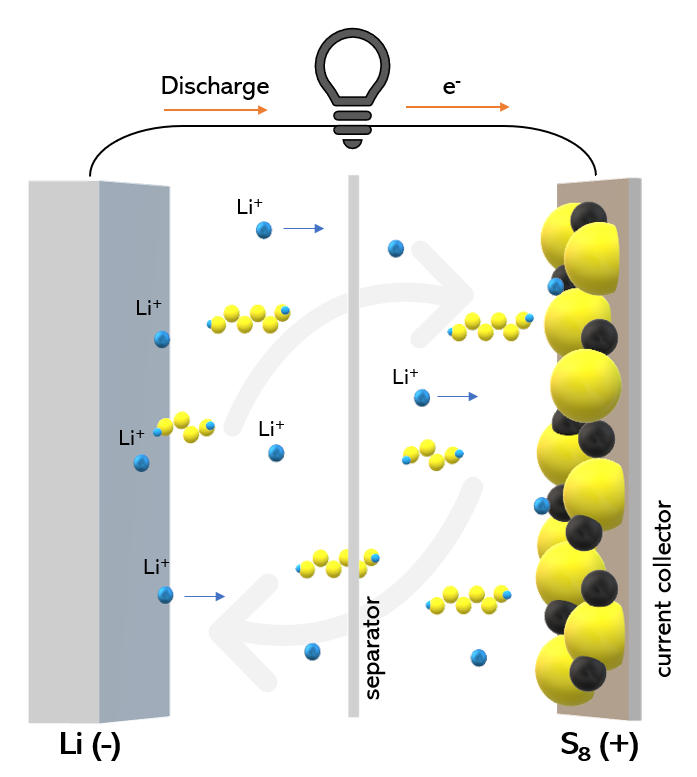
In their study titled, “Solvent selection criteria for temperature-resilient lithium-sulfur batteries.” published in Proceedings of the National Academy of Sciences (PNAS), researchers have developed an electrolyte made of a liquid solution of dibutyl ether mixed with a lithium salt. Due to weak binding between molecules of dibutyl ether and lithium ions, the electrolyte molecules easily part ways from lithium ions as the battery runs. This weak interaction improves battery performance at low temperatures, as shown in previous research. Also, dibutyl ether can take a relatively higher heat as it stays liquid at high temperatures because of its boiling point of 141°C. In test runs, these batteries have retained 87.5% and 115.9% of their energy capacity at -40 and 50 °C (-40 and 122 F), respectively. Additionally, their high columbic efficiencies of 98.2% and 98.7% at these temperatures have ensured that these batteries can undergo more charge and discharge cycles before they stop working.
“You need a high-temperature operation in areas where the ambient temperature can reach the triple digits and the roads get even hotter. In electric vehicles, the battery packs are typically under the floor, close to these hot roads,” said Zheng Chen, a professor at the UC San Diego, and senior author of the study. He further explained, “Also, batteries warm up just from having a current run through during operation. If the batteries cannot tolerate this warm-up at high temperature, their performance will quickly degrade.”
The electrolyte developed by the research group is compatible with a lithium-sulfur battery with lithium and sulfur as anode and cathode, respectively. Lithium-sulfur batteries have high energy densities and lower costs. They can store up to two times more energy per kilogram than today’s lithium-ion batteries. Their combination with the developed electrolyte further enhances the feature of the batteries. Such batteries tested by the group also had much longer cycling lives than a typical lithium-sulfur battery. “Our electrolyte helps improve both the cathode side and anode side while providing high conductivity and interfacial stability,” said Chen. The group continues their work to optimize these batteries for even higher temperatures and extend their life cycle.


Leave A Comment