Chemical elements combine to form crystals in a periodic way where the atoms are arranged in a periodic lattice of points with a limited set of symmetries, has been a basic belief for more than two centuries.
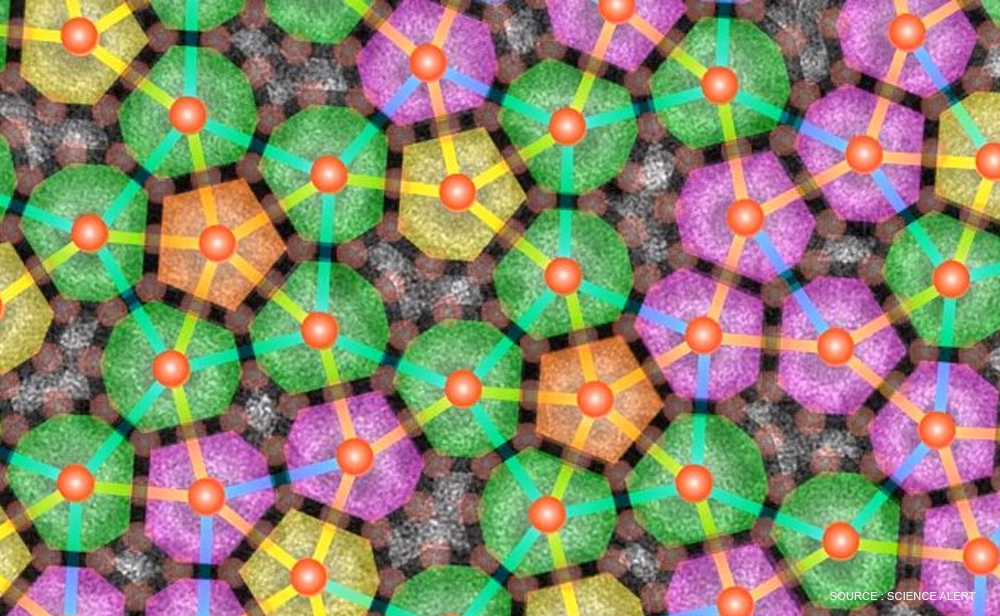
A covering equivalent to a single Penrose tiling can be constructed using a single decagonal tile if two kinds of overlapping regions are allowed. This idea was developed by Roger Penrose in 1972 for a two dimensional case and later extended to 3D case. A quasi-periodic crystal, or quasicrystal, is a structure that is ordered but not periodic.
A quasi-crystalline pattern can continuously fill all available space, but it lacks translational symmetry. While crystals, according to basic sciences, can possess only two-, three-, four-, and six-fold rotational symmetries, the Bragg diffraction pattern of quasicrystals shows sharp peaks with five fold symmetry order.
While their structure and formation are of immense basic interest, quasicrystals also show unique physical and chemical properties that make them promising for technological applications. The electrical and thermal conductivities of the best ordered quasicrystals are surprisingly low, similar to semiconductors than to metallic alloys.
All quasicrystals are hard and brittle. Zirconium–Nickel quasicrystals can store prodigious quantities of hydrogen, up to two hydrogen atoms for each metal atom, presumably due to the large number of tetrahedral sites in the quasi-lattice, and favorable alloy chemistry.
It is a material available at low cost and the relatively rapid absorption and desorption of Hydrogen at temperatures between 300°C and 350°C, make these systems promising for hydrogen storage applications


Leave A Comment